Pharmaceutical secondary packaging with banding
Rethinking your approach to pharmaceutical secondary packaging?
Banded packaging offers a smart, sustainable way to bundle and label products in one streamlined solution.
Less material. Less waste. More efficiency.
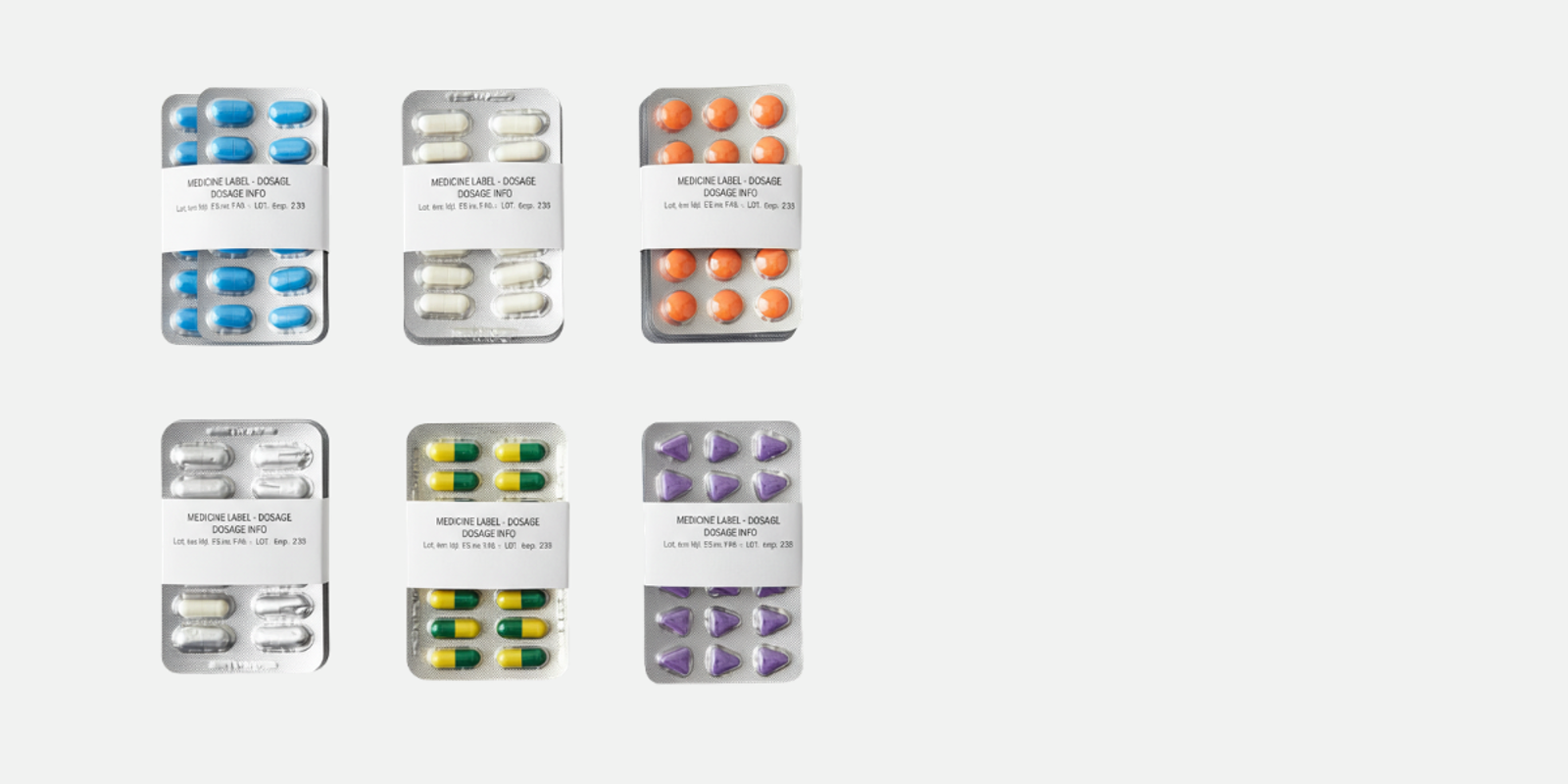
What is pharmaceutical secondary packaging?
Pharmaceutical secondary packaging refers to all packaging applied around a product’s primary packaging. The role is to bring these individual units together, provide essential product information, and prepare them for safe transport, storage, and retail distribution.
The role of banding in pharmaceutical secondary packaging
Banding works as a secondary packaging solution by applying banding material around one or multiple items and sealing it.
The result? An efficiently bundled stack with minimal material use.
Banded packaging provides:
- Product grouping and unitisation
- Clear identification and information display
- Secure handling during logistics
- Efficient end of line automation


Banded pharmaceutical packaging
Banded packaging offers a flexible solution suitable for a wide range of applications.
Common examples include:
- Bundling multiple blister packs together
- Securing cartons or boxes
- Grouping vials or syringes for distribution
- Adding product or batch identification
The banding material holds everything firmly together, providing a secure seal with clear tamper evidence for added safety and confidence.
A better alternative for pharmaceutical secondary packaging
Traditional packaging uses lots of material and slows down or complicates your packaging line. Banding offers a smarter alternative.
With banding, you can:
- Uses significantly less packaging material
- Supports tamper evidence
- Integrate seamlessly into your existing packaging line
- Adapt quickly to different product formats
Banded packaging simplifies secondary packaging while maintaining control, compliance, and efficiency.


Compliance and traceability in pharmaceutical packaging
Meet strict regulatory requirements for identification, traceability, and serialization by printing variable information directly onto the banding material.
With banding, you can add:
- Batch and lot identification
- Barcodes and data matrix codes
- Product information
The result? A streamlined pharmaceutical secondary packaging solution that delivers regulatory confidence.
Automated pharmaceutical secondary packaging
Bandall banding machines are built to fit seamlessly into your packaging line, whether at the end of the line or fully integrated inline.
Our options include:
- Stand-Alone units for small-scale packaging
- Automatic banding systems for higher speeds
- Modular machines for seamless in-line integration
- Custom-engineered projects for unique requirements
With efficient and reliable banding technology, you keep your operations agile, streamlined, and ready for full automation.


Sustainable pharmaceutical secondary packaging
Banded packaging supports sustainable pharmaceutical secondary packaging by:
- Minimising overall material usage
- Supporting paper-based banding materials
- Reducing reliance on excessive plastic films or cardboard
- Improving recyclability
Achieve your sustainability goals with a smarter, lower-impact solution.
Why choose banding for pharmaceutical secondary packaging
Banded packaging brings efficiency, control, and flexibility together in one streamlined solution. Applied around primary pharmaceutical packaging, it supports compliance, automation, and sustainability without adding unnecessary packaging layers.
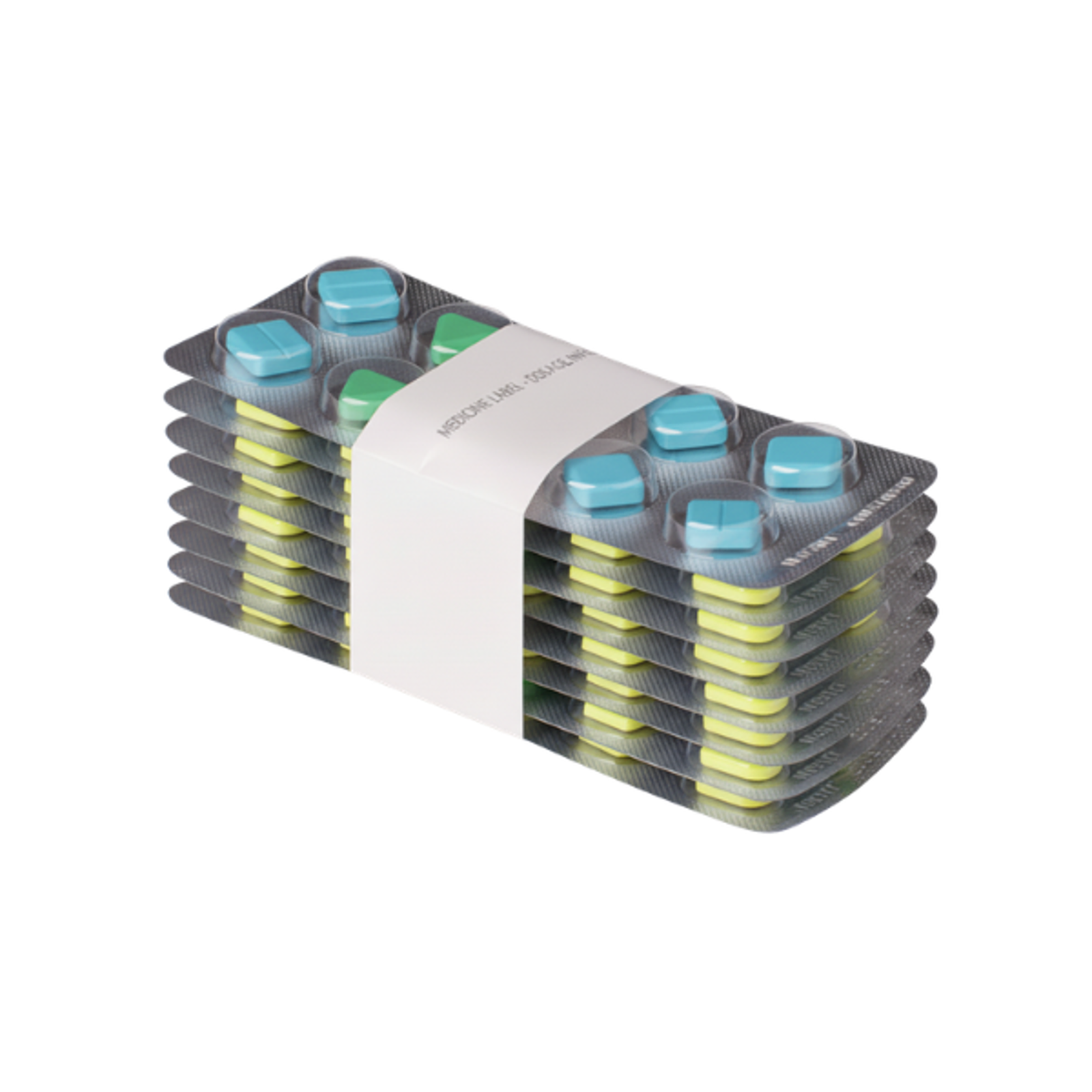

Use Case: GTE
Streamlining Sealing and Communication with Banding
For GTE Engineering, it's clear: banding is a valuable secondary packaging solution in the pharmaceutical industry. As a specialist in custom pharmaceutical packaging lines, GTE relies on banding technology to deliver efficient bundling and labeling within fully integrated systems.
Discover how they used banding to solve a specific customer challenge, securely bundling and labeling glass vials in a plastic tub while improving efficiency and maintaining compliance.